Hi everyone, I’m Justin here. Sorry for my super late post. For the last post, I’ll write about slide making.
Slide making involves dropping fixed cell suspension onto slides. Slides are then air dried. The air drying is based on the principle that fixed cells are supported with a thin layer of fixative. When the fixative evaporates, the cell would be pressed down, resulting in the spreading of chromosomes. The rate of fixative evaporation has to be controlled. If evaportation is too fast, a cytoplasmic background will be present and spreading will be poor. If the fixative evaporates too slowly, the weakened cell membrane may lose its integrity, leading to chromosome loss. Thus, the slide making process is able to eventually influence chromosome spreading and morphology.
The main factors that affect the slide making process are temperature, humidity and airflow. Humidity relates to the amount of moisture in the air. If the air is dry, evaporation rate will be faster, while if the air is moist, evaporation rate will be slower. Environment temperature is taken into account together as warm air is able to hold more moisture. The airflow within the area that slides are made affects the speed of drying. These factors directly affect the drying process, thus being the most important factors in a successful slide.
The lab I’m in uses a de-humidifier and the air conditioner for temperature and humidity. Another lab has a slide drying chamber, in which temperature, humidity, as well as air flow can be controlled more easily. It is important to consistently get slides of appropriate quality.
That’s all. Take care. To SIP and MP !
Ng Tze Yang Justin
0703747F
Sunday, October 25, 2009
Sunday, October 18, 2009
Mammalian Cell Technology
For my major project, I am required to culture and subculture mammalian cells. The type of cell I am culturing is large lung cancer cells; NCI-H460. This cell line grows best in RPMI medium.
Culturing of NCI-H460 cell line
1. Cryovial containing 1ml of NCI-H460 cells are removed from the liquid nitrogen and thawed in the 37°C water bath.
(Ensure that the cryovial is securely capped to prevent any leakage.)
2. The RPMI medium is warmed at 37°C water bath for approximately 30 minutes.
3. 5ml of the RPMI medium is added into the centrifuge tube.
4. All cells are removed from the cryovial and added into the 5ml RPMI medium.
5. The centrifuge tube is centrifuged at 1000rpm for 5 minutes.
6. The supernatant is removed and discarded into sodium hypochlorite.
7. The cell pellet is suspended in 10ml of RPMI medium.
8. 50µl of the cell suspension is removed and placed into a microcentrifuge tube (for cell counting).
9. The remaining cell suspension is placed into a T-75 flask.
10. 10ml of RPMI medium is added into the T-75 flask.
11. Incubate at 37°C with 5% CO₂.
Cell counting to determine seeding density
1. 50µl of the cell suspension is added into 50µl of trypan blue.
(Trypan blue will stain the dead cells)
2. Mix well by pipetting it up and down slowly.
(Ensure that the tip of the pipette is not placed against the surface to prevent damaging the cells.)
3. 10µl of the mixture is pipetted into the haemocytometer chamber.
4. Viable cells within the four corner squares of the haemocytometer are counted.
5. The cell density is calculated, average number of cells X 2 X 10
6. The total number of viable cells is calculated, cell density X 10ml
7. Clean the haemocytometer with alcohol.
(Ensure that it is not left for too long as the trypan blue will stain the haemocytometer.)
Strict aseptic techniques need to be observed as presence of contamination will prevent the growth of mammalian cells.
Liyana
0703827F
Culturing of NCI-H460 cell line
1. Cryovial containing 1ml of NCI-H460 cells are removed from the liquid nitrogen and thawed in the 37°C water bath.
(Ensure that the cryovial is securely capped to prevent any leakage.)
2. The RPMI medium is warmed at 37°C water bath for approximately 30 minutes.
3. 5ml of the RPMI medium is added into the centrifuge tube.
4. All cells are removed from the cryovial and added into the 5ml RPMI medium.
5. The centrifuge tube is centrifuged at 1000rpm for 5 minutes.
6. The supernatant is removed and discarded into sodium hypochlorite.
7. The cell pellet is suspended in 10ml of RPMI medium.
8. 50µl of the cell suspension is removed and placed into a microcentrifuge tube (for cell counting).
9. The remaining cell suspension is placed into a T-75 flask.
10. 10ml of RPMI medium is added into the T-75 flask.
11. Incubate at 37°C with 5% CO₂.
Cell counting to determine seeding density
1. 50µl of the cell suspension is added into 50µl of trypan blue.
(Trypan blue will stain the dead cells)
2. Mix well by pipetting it up and down slowly.
(Ensure that the tip of the pipette is not placed against the surface to prevent damaging the cells.)
3. 10µl of the mixture is pipetted into the haemocytometer chamber.
4. Viable cells within the four corner squares of the haemocytometer are counted.
5. The cell density is calculated, average number of cells X 2 X 10
6. The total number of viable cells is calculated, cell density X 10ml
7. Clean the haemocytometer with alcohol.
(Ensure that it is not left for too long as the trypan blue will stain the haemocytometer.)
Strict aseptic techniques need to be observed as presence of contamination will prevent the growth of mammalian cells.
Liyana
0703827F
Monday, October 12, 2009
ASOT
ASOT is commonly ordered by doctors whom patient is suspected of having Group A Streptococcus infection. Procedure for ASOT is as followed.
1. A drop of positive control was dispensed onto the 1st test area.
2. A drop of negative control was dispensed onto the 2nd test area.
3. A drop of sample was dispensed onto the 3rd test area using micropipette.
4. A drop of latex reagent was added to the 3 test areas.
5. Sample and reagent were mixed thoroughly using wooden sticks.
6. Test card was placed on electronic rotator for 2 minutes at 100 ± 2 rpm.
7. Test results was observed and recorded.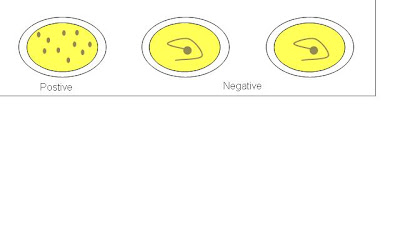

A negative ASOT result does not rule out the possibility of patient having Group A Streptococcus infection. If patient is still suspected of having the infection, a second blood sample should be taken for the test four weeks later from the previous ASOT test day.
Hui Juan
0702012F
Sunday, October 4, 2009
NNJ
In this posting, i'll be talking about NNJ investigation due to ABO incompatibility between the mother and baby.
NNJ - Neonatal Jaundice
Ok. For this investigations, it will be conducted in the blood bank. The nurses will send in a EDTA baby sample and 1 plain tube containing the mother's blood and another EDTA tube containing the mother's blood. So we have 1 tube from the baby, usually the cord blood and another 2 from the mother. These 3 tubes will be sent in a biohazard bag that is attached to the request form.
Mostly NNJ is caused by ABO incompatibility. So the first step is to test for baby and mother's blood group and check if there's incompatibility.
So, if the mother is Group O/A and the baby is group AB/B, there is blood group incompatibility. (Mother has anti-B)
If the mother is Group AB and the baby is group O, there's no blood group incompatibility.
For the baby's sample we only do forward grouping. Reason being: they haven't develop antibody yet.
After we determine that there is ABO incompatibility, we proceed to the "antibody titration stage" We either do the anti-A or anti-B titration with freshly prepared group A or B cells. Like erm, if the mother is Group A, baby group B, she has anti-B, so we do anti-B titration.
It's not possible that both Anti-A and Anti-B titration is required, because if the mother is group O, how can baby be of group AB? Unless the baby is not hers?!
Right. So for the titration we label them from 1:1 dilution to 1:1024 dilution.
1. 3 drops of saline is added into all but 1st tube
2. Into tubes "1:1" and "1:2", we drop 3 drops of mother's serum(plain tube)
3. From tube "1:2" to "1:4" transfer 3 drops over after mixing
4. From tube "1:4" to "1:8" transfer 3 drops over after mixing
5. Keep doing this until the last tube where 3 drops will be pipetted away(from steps 1 to 5, don't wash the pipette. Just keep using the same pipette. This is to avoid further dilution.
6.Then, go over to the fridge, take out a unit of blood of A packed cell(for anti-A titration).
Pull out a segment of blood from the unit.
 See the "tubing-like" rubber structure containing blood, there's a machine which can actually like fuse part of the "tube" together to create a segment, so we can actually pull out a segment without having to puncture and dirty the blood.
See the "tubing-like" rubber structure containing blood, there's a machine which can actually like fuse part of the "tube" together to create a segment, so we can actually pull out a segment without having to puncture and dirty the blood.
7. So to remove impurities, we wash it in 0.9% saline once
8. Then dilute the cells to create a 3-5% cell suspension.
9. Pipette a drop of the cell suspension into all tubes, from 1:1 to 1:1024
10. Incubate all the tubes for 1 hour
11. Centrifuge at low speed, 1000rpm for 15seconds
Read the results. Usually the first 2-3 tubes will be fully haemolysed.
In some tubes, there'll be partial haemolysis, with some agglutinates still present while in some. Record the results. Notify the ward staff/doctor if haemolytic anti-A or anti-B is detected
Thank you!
yanhong 0703979e
NNJ - Neonatal Jaundice
Ok. For this investigations, it will be conducted in the blood bank. The nurses will send in a EDTA baby sample and 1 plain tube containing the mother's blood and another EDTA tube containing the mother's blood. So we have 1 tube from the baby, usually the cord blood and another 2 from the mother. These 3 tubes will be sent in a biohazard bag that is attached to the request form.
Mostly NNJ is caused by ABO incompatibility. So the first step is to test for baby and mother's blood group and check if there's incompatibility.
So, if the mother is Group O/A and the baby is group AB/B, there is blood group incompatibility. (Mother has anti-B)
If the mother is Group AB and the baby is group O, there's no blood group incompatibility.
For the baby's sample we only do forward grouping. Reason being: they haven't develop antibody yet.
After we determine that there is ABO incompatibility, we proceed to the "antibody titration stage" We either do the anti-A or anti-B titration with freshly prepared group A or B cells. Like erm, if the mother is Group A, baby group B, she has anti-B, so we do anti-B titration.
It's not possible that both Anti-A and Anti-B titration is required, because if the mother is group O, how can baby be of group AB? Unless the baby is not hers?!
Right. So for the titration we label them from 1:1 dilution to 1:1024 dilution.
1. 3 drops of saline is added into all but 1st tube
2. Into tubes "1:1" and "1:2", we drop 3 drops of mother's serum(plain tube)
3. From tube "1:2" to "1:4" transfer 3 drops over after mixing
4. From tube "1:4" to "1:8" transfer 3 drops over after mixing
5. Keep doing this until the last tube where 3 drops will be pipetted away(from steps 1 to 5, don't wash the pipette. Just keep using the same pipette. This is to avoid further dilution.
6.Then, go over to the fridge, take out a unit of blood of A packed cell(for anti-A titration).
Pull out a segment of blood from the unit.
 See the "tubing-like" rubber structure containing blood, there's a machine which can actually like fuse part of the "tube" together to create a segment, so we can actually pull out a segment without having to puncture and dirty the blood.
See the "tubing-like" rubber structure containing blood, there's a machine which can actually like fuse part of the "tube" together to create a segment, so we can actually pull out a segment without having to puncture and dirty the blood.7. So to remove impurities, we wash it in 0.9% saline once
8. Then dilute the cells to create a 3-5% cell suspension.
9. Pipette a drop of the cell suspension into all tubes, from 1:1 to 1:1024
10. Incubate all the tubes for 1 hour
11. Centrifuge at low speed, 1000rpm for 15seconds
Read the results. Usually the first 2-3 tubes will be fully haemolysed.
In some tubes, there'll be partial haemolysis, with some agglutinates still present while in some. Record the results. Notify the ward staff/doctor if haemolytic anti-A or anti-B is detected
Thank you!
yanhong 0703979e
Subscribe to:
Posts (Atom)